Abstract
Background
The use of cannabis has been clinically associated with decreased motivation to engage in normally rewarding activities. However, evidence from previous controlled studies is mixed.
Method
In this study, we examined the effects of acute delta-9-tetrahydrocannabinol (THC) versus placebo on a task measuring willingness to exert effort for rewards. This is a secondary analysis of a larger study examining interactions between ovarian hormones and THC. In this within-subjects study, oral THC and placebo were administered under double-blind conditions in counterbalanced order to healthy young adult (M age = 24 years) women with previous cannabis experience who were not regular users. Forty subjects completed three 4-h sessions with PL, 7.5 and 15 mg THC, while an additional 18 completed only PL and 15 mg THC sessions (design abridged due to pandemic). At each session, they completed a task consisting of making repeated choices between a hard and an easy task, which were worth varying amounts of money at varying probabilities.
Results
THC dose-dependently decreased hard task choices (drug effect, b = − 0.79, SE = 0.29, z = − 2.67, p < 0.01), especially at moderate to high expected values of reward (drug × probability × amount interaction, b = 0.77, SE = 0.38, z = 1.99, p = 0.04). THC also slowed task performance (drug effect, b = 0.01, SE = 0.005, t(5.24) = 2.11, p = 0.04), but the effect of THC on choice was still significant after controlling for this psychomotor slowing.
Conclusions
These findings support the idea that cannabis acutely reduces motivation to earn non-drug rewards. Still to be determined are the neurochemical mechanisms underlying this effect.
Similar content being viewed by others
Introduction
It has long been believed that cannabis use is associated with apathy and a lack of motivation, a constellation of behaviors sometimes referred to as the “amotivational syndrome” (Volkow et al. 2016; Petrucci et al. 2020). The evidence for this syndrome is mixed, with some studies finding increases in apathy or anhedonia in regular cannabis users (Barnwell et al. 2006; Cherek et al. 2002; Lac 2018; Pacheco-Colón et al. 2018), while others do not (Barnwell et al. 2006). It is difficult to define and measure the syndrome, and if it exists, it is difficult to determine the direction of the association. That is, it is not clear whether lower motivational states result from the use of cannabis and its associated lifestyle, or if they pre-dated and perhaps contributed to the use of cannabis (Leventhal et al. 2017). It is also not clear if this syndrome refers mainly to long-term consequences of chronic cannabis use or whether it also reflects an acute behavioral effect of cannabis. “Amotivational syndrome” typically refers to a lasting pattern of apathy and lack of motivation attributed to repeated cannabis use, but it may also reflect the direct pharmacological effects of acute cannabis consumption on motivation or willingness to exert effort.
Several studies have examined the direct, acute effects of cannabis or its primary active ingredient delta-9-tetrahydrocannabinol (THC) on effort or motivated behavior in humans. In one early inpatient research study (Foltin et al. 1990), regular cannabis users were given the opportunity to earn rewards either under the influence of smoked cannabis (2.7% THC) or after placebo. Contrary to expectations, cannabis increased, rather than decreasing, the subjects’ willingness to work for desired outcomes. Cherek et al. (2002) tested the effects of smoking cannabis cigarettes (1.77% or 3.58% THC) on participants’ willingness to exert effort using a progressive ratio procedure where subjects were required to emit increasing numbers of button presses to earn money. Cannabis decreased the number of presses subjects emitted, especially when the monetary value of the reward was low. However, in the Cherek et al. study, as in many other studies using tasks involving motor performance to assess motivation, it is difficult to separate drug effects on motor performance from the effects on motivation (Salamone et al. 2015).
Procedures have been developed in rodents to distinguish between the effects of drugs on motor performance versus motivation (Hosking et al. 2015), often to study the role of dopamine in motivated behaviors (Salamone et al. 1994). In these procedures, animals choose between high or low effort (either physical or cognitive) responses to earn rewards (Salamone 2009). The researchers also assess motor performance to separate motivational effects from motoric effects (Salamone et al. 2016). One study using such a procedure in rodents, assessed the effects of THC (0, 0.3, 1, 2, 3 mg/kg THC) on cognitive effort (Silveira et al. 2017). The study found that THC decreased preference for hard trials without impairing the animals’ ability to accurately complete them.
Similar procedures have been used with human participants (Wardle et al. 2011), including one study investigating the effects of vaporized cannabis in regular cannabis users (Lawn et al. 2016). Lawn et al. used an effort choice task to study the effects of vaporized cannabis (8 mg THC) or placebo on a task in which subjects chose between performing a high-effort task (finger tapping) for money, or a low effort task for a lower amount of money (Treadway et al. 2009). Cannabis decreased the choices for the high effort option. In a second phase of the study, the investigators compared performance on the effort task in occasional users and dependent cannabis users to assess lasting effects on motivation. The occasional and dependent users did not differ in their selection of high versus low effort choices. These findings provide some evidence that acute, but perhaps not chronic, administration of THC does decrease the willingness to exert effort.
The present study extends the findings of Lawn et al. (2016) in several ways. Instead of using inhaled cannabis, the present study examined the effects of oral THC, reducing the variability that might occur with inhalation and whole plant material. Second, we assessed two doses of THC (7.5 and 15 mg), to investigate dose-related effects. Another difference was that our study included only female participants. This is an artifact of the fact that this is a secondary analysis of a larger study investigating the interaction of ovarian hormones and THC, but nevertheless contributes to the literature on acute drug effects that have tended to include more male participants. Finally, our participants were lighter users of drugs and alcohol than the Lawn et al. subjects. Testing participants with little prior drug use minimize the potential influence of prior drug use on responses to the test drug. We hypothesized that THC would dose-dependently reduce the choice of the high effort option on the effort task (i.e., that there would be a linear main effect of the drug, such that effort in the PL condition < 7.5 mg condition < 15 mg condition). We additionally investigated interactions between the effect of the drug and the expected value of the reward (which is a combination of the amount of the reward and the probability of receiving the reward). Here our hypothesis was open. In previous studies, drug manipulations that increase effort have been more evident at low expected values (where overall effort is generally low; Soder et al. 2021). Thus any tendency of THC to decrease effort might be more evident in moderate to high expected values, where overall effort is generally high. However, Lawn et al. (2016) actually found more effects of cannabis at lower expected values. Thus, we explored these interactions primarily to inform future research by identifying what, if any, reward conditions on this task have greater sensitivity to the effect of THC.
Methods
Design
The study was initiated as a three-session, within-subjects counterbalanced design with placebo and two doses of THC (7.5 and 15 mg), but was later reduced to only two sessions: placebo and 15 mg THC. This change was made to minimize the number of sessions that needed to be conducted during the COVID-19 pandemic. We continued the higher dose only because interim analyses conducted at that point suggested that the effects of THC were (as expected) more evident at this dose. Therefore, 40 subjects completed all three sessions, and 58 completed two sessions. This is a secondary analysis of this study, with main results to be reported elsewhere. During the two or three 4-h laboratory sessions, participants completed the Effort Expenditure for Rewards Task (EEfRT; Treadway et al. 2009) after double-blind oral administration of 7.5 mg THC (early subjects only), 15 mg THC, and placebo, in a randomized order. Participants were women who reported having used cannabis at least four times throughout their lifetime but less than 11 times in the last 30 days.
Participants
Sixty healthy women (18–35 years) who reported some use of cannabis (> 4 times ever and < 11 times in the past month) participated. The screening included a semi-structured clinical psychiatric interview (First et al.) and a medical and drug use history. Exclusion criteria were prescription medication use, serious psychiatric disorders (psychosis, generalized anxiety disorder, major depressive disorder, severe post-traumatic stress disorder or obsessive–compulsive disorder, and moderate or severe substance use disorder), BMI < 19 or > 29, abnormal resting-state ECG or HR, or pregnant or planning to be pregnant. Participants agreed to refrain from alcohol and over-the-counter drug use for 24 h before and 12 h after the session, from cannabis use 7 days before and 24 h after the session, and from other recreational drugs 48 h before and 24 h after the session. Compliance was verified using breath (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA). The study was approved by the local institutional review board.
Forty individuals completed all three sessions, and 20 individuals completed only the placebo and 15 mg sessions. Two individuals were missing EEfRT data from the 15 mg THC session due to adverse reactions to the drug. Their available data were included in analyses, using multi-level modeling techniques that accommodate missing data. Excluding these two individuals did not change the pattern of results. Two other participants were excluded from all analyses of the EEfRT, as they chose the low effort task on all trials at all sessions (this is consistent with our exclusion criteria in previous uses of this task in repeated measures studies (Wardle et al. 2011; Soder et al. 2021). Therefore, all analyses reflect a sample size of 58.
Procedure
Participants attended a pre-study orientation session followed by two or three 4-h experimental sessions. During the orientation, participants provided informed consent, were familiarized with the study procedures, and practiced the EEfRT task. The experimental sessions were conducted in the early afternoon, separated by at least 3 days. Upon arrival, participants provided urine, blood, and breath tests to confirm drug abstinence, test for pregnancy, and examine hormone levels (not reported here). Heart rate (HR) and blood pressure (BP) were measured throughout the session (− 15, 30, 60, 90, 120, and 180 min post capsule; Omron 10 Plus, Omron Healthcare). After completing baseline drug effect questionnaires, subjects ingested a capsule containing THC (7.5 or 15 mg) or placebo under double-blind conditions. Participants completed drug effect questionnaires repeatedly throughout the session (− 15, 30, 60, 90, 120, and 180 min post capsule).
Drug
THC capsules (2.5, 5, and 10 mg; Dronabinol Akorn Inc, Lake Forest IL) were placed in opaque size 00 capsules with dextrose filler. Placebo capsules contained dextrose only.
Subjective drug effect measures
To assess cannabis-specific subjective drug effects, we included the Addiction Research Center Inventory (Martin et al. 1971; Chait et al. 1985). The ARCI consisted of 53 true–false questions measuring typical effects from several classes of drugs. The scores are the number of items endorsed. For our analysis, we focused on the M (marijuana) subscale which assesses cannabis (marijuana) effects. It includes 12 items such as “I have difficulty in remembering,” “My mouth feels very dry,” “I notice that my heart is beating faster,” and “My thoughts seem to come and go.” Scores range from 0 to 12 and specify the magnitude of cannabis-specific intoxication symptoms a participant was experiencing.
EEfRT task
The EEfRT (Treadway et al. 2009) is a multi-trial game in which participants are asked to choose on each trial between a “hard,” high effort/high reward task (HE/HR) and an “easy,” low effort/low reward task (LE/LR) to obtain varying monetary rewards (Fig. 1). The “hard task” (HE/HR) requires 100 button presses with the nondominant pinky finger within 21 s, and the “easy task” (LE/LR) requires 30 button presses with the dominant index finger within 7 s. For easy-task choices, subjects could win $1.00 for each successfully completed trial, and for hard-task choices, subjects could win higher amounts that varied per trial within a range of $1.24–$4.30 (“reward magnitude”). Trials were rewarded on a probabilistic basis. Some trials were “win” trials, in which the subject received the reward amount, while others were “no-win” trials, in which they did not receive money. Before each trial subjects were informed of the probability of receiving a reward, if they responded correctly: There were three levels of probability: “high” 88% probability of a winning trial, “medium” 50%, and “low” 12%. Probability levels were applied to both the hard and easy task, and there were equal proportions of each probability level across the experiment. Probability and reward information, task progress, and feedback displays (as depicted in Fig. 1) were presented on a computer screen. Button presses were completed on a standard keyboard.
Schematic diagram of a single EEfRT trial. A 1-s fixation cue; B 5-s choice period in which subjects are presented with reward magnitude of the hard task for that trial, and the probability of receiving a reward for that trial; C 1-s “ready” screen; D Subjects make rapid button presses to complete the chosen task for 7 s (easy task LC/LR) or 21 s (hard task HC/HR); E Feedback completion of the task; F Feedback on whether they received any money for that trial
Statistical analyses
Physiological and subjective drug effects
Physiological (HR, systolic BP, diastolic BP) and subjective effects (ARCI: M scale) of oral THC across session time were analyzed using multilevel models with Toeplitz covariance structures. Time (linear and quadratic) effects were included to allow for analysis of linear or curvilinear trends across time. All analyses were completed in IBM SPSS statistical software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Mixed-effects models offer significant advantages relative to traditional repeated-measures ANOVA in the handling of missing data, relaxation of assumptions of homogeneity of variance, and increased statistical power for smaller sample sizes. All physiological effects models (HR, systolic BP, diastolic BP) and subjective effects models (“feel drug,” M scale, state anxiety) included the effect of drug and time (linear and quadratic) effects as independent fixed variables. Significant linear effects of the drug were followed up by post hoc contrasts between placebo and 7.5 mg THC and placebo and 15 mg THC. Effect sizes are reported as unstandardized coefficients (B) with standard errors (SE).
EEfRT task
All analyses of the EEfRT task were performed in R (Team 2019) using lmer and lmertest packages with the Satterthwaite method for degrees of freedom (Bates et al. 2014; Kuznetsova et al. 2017). All continuous variables (i.e., amount and trial) were mean-centered and categorical variables (i.e., drug, probability, and session) were coded using polynomial contrasts to assess linear and quadratic effects. We established the random effects models by generating a maximal model and iteratively reducing it per Bates, Kliegl, Vasishth, and Baayen using the RePsychLing package (Bates et al. 2014; Baayen et al. 2015; Matuschek et al. 2017). Follow-up tests of significant main effects or interactions were conducted using the emmeans package (Lenth et al. 2019). Follow-up tests of interactions estimated the effect at each probability (12%, 50%, 88%) and two representative values of amount (low = $1.96, high = $3.40).
Choices were modeled using a generalized linear mixed-effects model (GLMM) with a logit link function for the binomial (hard/easy) outcome. Fixed effects were drug (placebo, 7.5 mg or 15 mg), probability (12%, 50%, or 88%), amount ($1.24–$4.21), and their interactions, with the probability by amount interaction representing the expected value of a reward. Fixed effects for trial number (0–50) and session (1, 2, or 3) were also entered into the equation to account for effects of fatigue and practice (Wardle et al. 2011). We also performed a separate analysis controlling for psychomotor speed. To do this, we first used a linear mixed-effects model to model key pressing speed as a function of the drug (placebo, 7.5 mg or 15 mg) and the type of task chosen (hard/easy). Individual estimates for the linear effect of the drug on tapping speed were entered as a between-subject covariate in the final choice model described above. Finally, we used GLMM to investigate if THC affected the participants’ ability to complete the tasks, with a logit link function for the binomial (completed, incomplete) outcome. This was a secondary analysis of this study, and as such a priori power calculations were not based on this outcome, nevertheless, our sample is considerably larger than previous investigations that have found acute effects of THC on motivation (e.g., N = 17 for Lawn et al. 2016, and N = 5 for Cherek et al. 2002).
Results
Participant characteristics
Demographic data for the participants can be found in Table 1. The participants who completed three sessions did not differ from participants who completed only two sessions.
Physiological measures
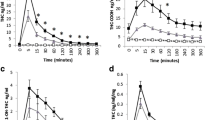
At both the 7.5 mg and 15 mg THC condition, HR significantly increased across session time compared to placebo (BTime*7.5 mg = 1.23, SE = 0.40, p = 0.002, 95% CI [0.45, 2.02]; BTime*15 mg = 2.40, SE = 0.37, p < 0.0005, 95% CI [1.67, 3.13]; Fig. 2). Follow up Bonferroni corrected t-tests comparing the drug to placebo at each time point indicated that the 15 mg dose increased HR at 60 min post-capsule and both the 7.5 mg and 15 mg dose increased HR from 90 to 180 min post-capsule. THC did not significantly affect systolic or diastolic BP across session time, at either dose, compared to placebo.
Effects of oral THC (7.5 mg, 15 mg) on mean heart rate in beats per minute across session time. Oral THC dose dependently increased heart rate across session time compared to placebo. Errors bars + / − 1 standard error. Bonferroni corrected post hoc t-tests: * p < 0.05 [placebo vs. 15 mg THC]; ^ p < 0.05 [placebo vs. 7.5 mg]
Subjective measures
At both doses, THC increased ARCI: M Scale scores across session time (BTime*7.5 mg = 0.71, SE = 0.07, p < 0.0005, 95% CI [0.57, 0.86]; BTime*15 mg = 0.91, SE = 0.07, p < 0.0005, 95% CI [0.78, 1.05]; Fig. 3). Follow-up Bonferroni corrected t-tests comparing drug to placebo at each time point indicated that the 15 mg dose increased ARCI: M scale scores at 60 min post-capsule and both the 7.5 mg and 15 mg dose increased ARCI: M scale scores from 90 to 180 min post-capsule.
Effects of oral THC (7.5 mg, 15 mg) on mean ARCI: Marijuana (M) scale across session time. Oral THC dose dependently increased ARCI: M scale scores across session time compared to placebo. Errors bars + / − 1 standard error. Bonferroni corrected post hoc t-tests: * p < 0.05 [placebo vs. 15 mg THC]; ^ p < 0.05 [placebo vs. 7.5 mg]
EEfRT task performance
Table 2 presents the raw % of hard task choices made under different drug, probability, and amount conditions. Table 3 presents the full results of the GLMM. As shown in Fig. 4, THC decreased overall choice of the hard task at the 15 mg dose (linear drug effect, b = − 0.79, SE = 0.29, z = − 2.67, p < 0.01). The 15 mg dose decreased hard choice tasks compared to placebo, with a 55% lower likelihood of selecting the hard choice task (OR = 0.45, SE = 0.13, z = − 2.71, p < 0.001), while the 7.5 mg dose was not significantly different from placebo (OR = 0.68, SE = 0.21, z = − 1.27, p = 0.21). Doses did not differ from one another (OR = 0.66, SE = 0.21, z = − 1.22, p = 0.18). Notably, the effect of THC depended on the expected value of the reward, which is a combination of the probability and amount of reward (linear drug × quadratic probability × amount interaction, b = 0.77, SE = 0.38, z = 1.99, p = 0.04). As shown in Fig. 5, there was no effect of oral THC at low expected values (low probability and low amount: OR = 1.03, SE = 0.58, z = 0.06, p = 0.96; low probability and high amount: OR = 0.56, SE = 0.25, z = − 1.32, p = 0.19). However, the effect of THC was more pronounced at moderate to high expected values, with 48–82% less likelihood of choosing the hard task (medium probability and low amount OR = 0.43, SE = 0.15, z = –2.41, p = 0.02; medium probability and high amount: OR = 0.40, SE = 0.13, z = − 2.91, p < 0.01, high probability and low amount OR = 0.52, SE = 0.20, z = –1.71, p = 0.08; high probability and high amount: OR = 0.18, SE = 0.08, z = − 4.07, p < 0.01). Figure 5 shows the effect of drug graphed at representative points across the range of possible reward amounts and at the three probabilities, with expected values for each point noted in parentheses. Although THC significantly and dose-dependently slowed key pressing speed (linear drug effect, b = 0.01, SE = 0.005, t(5.24) = 2.11, p = 0.04), including the effect of THC on keypress speeds as a covariate did not change the results of the choice analysis. This suggests that the observed decrease in hard task choices is not likely to be due to psychomotor slowing. THC also dose-dependently decreased the number of completed trials (linear drug effect, OR = 0.26, b = − 1.36, SE = 0.33, z = − 4.11, p < 0.01), with a 74% lower likelihood of completion.
Effects of oral THC on % hard choice task by the probability of win and reward amount. Expected values are displayed in parentheses under each reward amount. Interaction between drug, probability, and reward amount demonstrated that the drug effect was generally more pronounced at medium to high expected values
Discussion
We found that oral administration of THC decreased willingness to exert effort for rewards compared to placebo. This effect appeared dose-dependent, as was a significant linear effect of the drug on effort, and post hoc tests showed the effect of the drug on effort was only significant at 15 mg; however, it should be noted that the 7.5 mg dose did not differ significantly from either PL or 15 mg. The effects of THC on effort for reward were also more pronounced at moderate to high expected values. THC also slowed the performance of the task overall, but the main effect of THC on motivation and the moderation of this effect by expected value was still significant after controlling for psychomotor slowing. THC also significantly reduced the number of completed tasks, an effect that could be due to both psychomotor and motivational effects of THC.
Our primary results are consistent with findings from a previous study testing effects of vaporized cannabis with and without cannabidiol (CBD) on this same task (8 mg; Lawn et al. 2016). Lawn et al (2016) vaporized cannabis without CBD; the condition most similar to the oral THC used here also reduced willingness to exert effort for rewards. In this previous study, the inclusion of CBD somewhat (although not significantly) ameliorated the effects of cannabis on effort. We are unable to speak to combining CBD with THC, but our study clearly supports a dose-dependent detrimental effect of THC alone on effort. There were also some differences in results. First, Lawn et al. found that effects of THC were more pronounced at low probability. In contrast, we saw no effects of THC on low probability/low expected value trials. Instead, the effects of THC were more pronounced at moderate to high expected values. This could simply represent the fact that effect estimates for interactions have naturally lower power and may produce less stable and replicable results. However, we also saw pronounced effects on the psychomotor speed that were not present in Lawn et al. These psychomotor effects were not responsible for the observed decrease in effort, suggesting separate effects of THC on psychomotor versus decision-making processes. We may have seen psychomotor effects while Lawn et al. did not due to the use of a mixed-gender sample in the Lawn and colleagues study, contrasted with our all-female population or due to pharmacodynamic or pharmacokinetics of the drug administration (vaporized cannabis vs. oral THC, dose). These results could also indicate that tolerance for the psychomotor effects of THC develops more quickly than tolerance for decision-making effects. This would explain why the drug failed to impair psychomotor performance in the heavier users Lawn and colleagues sampled, while still affecting decision-making in that study. Confirming this possibility would require a longitudinal study of cannabis users over the course of the development of tolerance. Finally, we also saw significant effects of THC on the likelihood of completing tasks. This could be due to either psychomotor effects hindering completion of trials, or to motivational effects leading participants to “abandon” trials. This decrease in task completion was also not observed in the Lawn study, again suggesting potentially “stronger” effects of THC here, either due to our lighter-using sample, due to dose-dependent effects being more evident at our higher dose, or due to our differing route of administration.
Our study adds to evidence that the endocannabinoid system is involved in computations involving effort and reward. Like all drugs of abuse, cannabis increases dopamine levels in the nucleus accumbens (NAcc) (Zehra et al. 2018), which would generally be expected to increase willingness to exert effort (Di Chiara 1988; Salamone et al. 2007). However, it is true that the effect of cannabis on dopamine is weaker than other classic drugs of abuse (Bossong et al. 2014), which might also justify a null effect on effort. However, both our study and previous studies in humans suggest cannabis actually impairs willingness to exert effort for rewards. Indeed, cannabinoid agonists administered either directly into the NAcc, the anterior cingulate cortex, or the orbitofrontal cortex of rodents (Fatahi 2018), decrease willingness to exert effort for reward. The small number of fMRI studies involving THC administration and reward measures that have been conducted in humans similarly suggest some dampening of reward-related responses, including in the NAcc during anticipation of monetary reward, and in the ventral striatum during the enjoyment of music (see Skumlien et al. 2021 for a recent review). This has led to the suggestion that the endocannabinoid system may function as a “brake” on effort-based decision-making, although the exact details of how this endocannabinoid “brake” interacts with the dopaminergic aspects of effort-based decision-making are unknown (Fatahi 2018). Moreover, it is not clear precisely how THC, as a partial agonist, affects the endocannabinoid receptor system. The downstream effects of a partial agonist such as THC are difficult to predict. On one hand, the partial agonist may activate the receptor but do so with less efficacy (i.e., lesser maximal effect) than a pure agonist. On the other hand, a partial agonist may also block the activity of an endogenous ligand, thus dampening downstream actions of the system. Adding to the complexity, the endocannabinoid system modulates actions of other neurotransmitters, in ways that are difficult to predict. Thus, it is difficult to speculate about the receptor mechanisms that underlie the effects of THC on higher-level cognitive behaviors such as effort. Finally, motivation for rewards is also underpinned by not just dopamine, but a complex set of interlocking systems with adenosine elements (Salamone et al. 2010) and opioidergic elements (Laurent et al. 2015) among many others, any of which could be indirectly altered by THC administration.
The cognitive or psychological effects underlying this change in motivation for reward also remain to be elucidated. Typical cannabis effect scales, including the one used here, contain items such as “Things around me seem more pleasing than usual” and “I feel as if something pleasant had just happened to me,” so it is possible cannabis satisfies some need for reward, reducing motivation for other, non-drug rewards. However, stimulants such as amphetamine also produce euphoria, yet increase exertion of effort for other rewards (Wardle et al. 2011; Soder et al. 2021). It is also possible that cannabis operates on perceptions of effort, making costs appear higher rather than rewards lower. It seems likely that these changes in perception of effort may occur independently of actual psychomotor changes in ability. Indeed, the effects of amphetamine on motivation for reward are independent of psychomotor speeding (Wardle et al. 2011; Soder et al. 2021), just as the effects of cannabis appear independent of psychomotor slowing. Further, caffeine results in psychomotor speeding without affecting choice behavior in this same task (Wardle et al. 2012). Future studies should consider including measures of perceived effort to investigate this possibility further.
It is also important to consider that the amotivational effects of THC may be specific to reward type. In mice, systemically administered THC did not alter motivation to engage in wheel running (typically a rewarding activity in mice) but actually increased motivation for food reward (Hurel et al. 2021). This raises a point nearly universal to translational research, which is that rodent studies are typically conducted using primary rewards, such as food, while human studies typically use secondary rewards, such as money. The neural circuitry underlying primary and secondary reward does seem to differ (Sescousse et al. 2013), suggesting they could be differently influenced by pharmacological manipulations. Future human behavioral pharmacology studies should consider contrasting effects of drugs on standard money-based effort tasks with directly parallel tasks that use food rewards (Racine et al. 2019).
This study had several limitations. First, our sample consisted entirely of women, so we were unable to test for any moderating effects of sex on these results. Our primary results were similar to a previous study that used a mixed-gender sample (Lawn et al. 2016), but a direct comparison of sexes within the same design would be preferable. Second, although oral use of cannabis and THC is not uncommon, smoked administration remains the most common route in the US and internationally (Freeman 2020). Smoked versus oral administration may have different subjective and behavioral effects due to pharmacokinetic factors (Wachtel et al. 2002). Similarly, we used only THC in this study, omitting the other cannabinoids present in whole-plant cannabis. Although some studies suggest that whole-plant cannabis has similar effects to THC alone (Wachtel et al. 2002), there is some evidence that CBD, which is found in whole-plant cannabis and some medical preparations of THC (e.g., Sativex, GW Pharmaceuticals) reduce the amotivational effects of THC (Lawn et al. 2016; Silveira et al. 2017). Future studies should continue to explore the possibility that CBD may reduce the harms of THC in this area. Finally, we were unable to examine the dose–response curve with full power due to abbreviations made in the protocol in response to COVID-19, with only 40 of our 58 participants completing both doses of THC.
In summary, our study adds to the evidence suggesting that THC has dose-dependent acute amotivational effects. Although this study does not speak to the possibility of chronic or lasting effects of THC, more frequent or heavier users of cannabis could certainly experience these effects in everyday life. This could contribute both to qualitative impressions of a “amotivational” presentation in cannabis users, and to the mixed quantitative data, assuming these effects are primarily acute, and present at higher doses. The information that cannabis seems to have acute impacts on motivation, particularly at higher doses, is important for providers who are counseling individuals with cannabis use disorder about potential problems associated with their use, and for individuals who are considering either abstaining from cannabis or changing their use to reduce associated harms.
References
Baayen H, Bates D et al. (2015). RePsychLing: data sets from psychology and linguistics experiments. R package version 0.0 4
Barnwell SS, Earleywine M, Wilcox R (2006) Cannabis, motivation, and life satisfaction in an internet sample. Substance Abuse Treatment, Prevention, and Policy 1(1):2. https://doi.org/10.1186/1747-597X-1-2
Bates D, Mächler M et al. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823
Bossong MG, Jansma JM, Bhattacharyya S, Ramsey NF (2014) Role of the endocannabinoid system in brain functions relevant for schizophrenia: an overview of human challenge studies with cannabis or ∆9-tetrahydrocannabinol (THC). Prog Neuropsychopharmacol Biol Psychiatry 52:53–69
Chait L, Fischman M.W et al. (1985) ‘Hangover’effects the morning after marijuana smoking. Drug and alcohol dependence 15(3): 229-238.
Cherek DR, Lane SD et al (2002) Possible amotivational effects following marijuana smoking under laboratory conditions. Exp Clin Psychopharmacol 10(1):26
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci 85(14):5274–5278
Fatahi Z (2018) Activation of the cannabinoid system in the nucleus accumbens affects effort-based decision making. Pharmacol Biochem Behav 165:29–35
First M, Williams J et al. Structured clinical interview for DSM-5—research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association.
Foltin RW, Fischman MW et al (1990) Motivational effects of smoked marijuana: behavioral contingencies and low†probability activities. J Exp Anal Behav 53(1):5–19
Freeman T (2020) ‘Standard THC units’: a proposal to standardize dose across all cannabis products and methods of administration. Addiction 115(7): 1207-1216.
Hosking JG, Floresco SB et al (2015) Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology 40(4):1005–1015
Hurel I, Muguruza C et al (2021) Cannabis and exercise: effects of Δ9-tetrahydrocannabinol on preference and motivation for wheel-running in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry 105:110117
Kuznetsova A, Brockhoff PB et al (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82(13):1–26
Lac A (2018) Testing the amotivational syndrome: marijuana use longitudinally predicts lower self-efficacy even after controlling for demographics, personality, and alcohol and cigarette use. Prev Sci 19(2):117–126
Laurent V, Morse AK, Balleine BW (2015) The role of opioid processes in reward and decision-making. Br J Pharmacol 172(2):449–459
Lawn W, Freeman TP et al. (2016) Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis ‘amotivational’hypotheses. Psychopharmacology 233(19): 3537-3552.
Lenth R, Singmann H et al (2019) Emmeans: estimated marginal means, aka least-squares means. R Package 1(3):4
Leventhal AM, Cho J, Stone MD, Barrington-Trimis JL, Chou C-P, Sussman SY, Riggs NR, Unger JB, Audrain-McGovern J, Strong DR (2017) Associations between anhedonia and marijuana use escalation across mid-adolescence. Addiction (abingdon, England) 112(12):2182–2190
Martin WR, Sloan J et al. (1971). Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clinical Pharmacology & Therapeutics 12(2part1): 245–258.
Matuschek H, Kliegl R et al (2017) Balancing type I error and power in linear mixed models. J Mem Lang 94:305–315
Pacheco-Colón I, Limia JM, Gonzalez R (2018) Nonacute effects of cannabis use on motivation and reward sensitivity in humans: a systematic review. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors 32(5):497–507
Petrucci AS, LaFrance EM et al (2020) A comprehensive examination of the links between cannabis use and motivation. Subst Use Misuse 55(7):1155–1164
Racine SE, Horvath SA et al (2019) Effort expenditure for rewards task modified for food: a novel behavioral measure of willingness to work for food. Int J Eat Disord 52(1):71–78
Salamone JD, Cousins MS et al (1994) Anhedonia or anergia? effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65(2):221–229
Salamone JD, Correa M et al (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191(3):461–482
Salamone JD, Correa M et al (2010) Role of dopamine–adenosine interactions in the brain circuitry regulating effort-related decision making: insights into pathological aspects of motivation. Future Neurol 5(3):377–392
Salamone JD, Koychev I et al (2015) Neurobiological basis of motivational deficits in psychopathology. Eur Neuropsychopharmacol 25(8):1225–1238
Salamone JD, Correa M et al (2016) The pharmacology of effort-related choice behavior: dopamine, depression, and individual differences. Behav Proc 127:3–17
Sescousse G, Caldú X et al. (2013) Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience & Biobehavioral Reviews 37(4): 681-696.
Silveira MM, Adams WK et al (2017) Δ9-Tetrahydrocannabinol decreases willingness to exert cognitive effort in male rats. Journal of Psychiatry & Neuroscience: JPN 42(2):131
Soder HE, Cooper JA et al (2021) Dose-response effects of d-amphetamine on effort-based decision-making and reinforcement learning. Neuropsychopharmacology 46(6):1078–1085
Team RC (2019). R Foundation for Statistical Computing. R: a language and environment for statistical computing
Treadway MT, Buckholtz JW et al. (2009) Worth the ‘EEfRT’? the effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One 4(8): e6598.
Volkow ND, Swanson JM et al (2016) Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiat 73(3):292–297
Wachtel S, ElSohly M et al (2002) Comparison of the subjective effects of δ 9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology 161(4):331–339
Wardle MC, Treadway MT et al (2011) Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci 31(46):16597–16602
Wardle MC, Treadway MT, de Wit H (2012) Caffeine increases psychomotor performance on the effort expenditure for rewards task. Pharmacol Biochem Behav 102(4):526–531. https://doi.org/10.1016/j.pbb.2012.06.016
Zehra A, Burns J et al (2018) Cannabis addiction and the brain: a review. J Neuroimmune Pharmacol 13(4):438–452
Funding
This research was supported by R01 DA002812. EP was supported by T32 DA043469 and F31 DA049391, MCW was supported by K08 DA040006, and HEW by F32 DA04854.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Cannabis and Cannabinoids
Rights and permissions
About this article
Cite this article
Wardle, M.C., Pabon, E., Webber, H.E. et al. Delta-9-tetrahydrocannabinol reduces willingness to exert effort in women. Psychopharmacology 239, 1487–1497 (2022). https://doi.org/10.1007/s00213-021-06032-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-06032-1